23.09.2025
From Preclinical Data to the Manufacture of a Clinical Batch
23.09.2025

Within medac CDMO, we have successfully completed the NaDiNa project, whose main objective was to develop a formulation, establish a manufacturing process, and prepare a prototype of a novel anticancer drug up to the stage enabling its clinical evaluation. The project was carried out in close collaboration with the Faculty of Medicine of Palacký University in Olomouc (UPOL) and was financially supported by the Technology Agency of the Czech Republic (TAČR).
The NaDiNa Medicinal Product
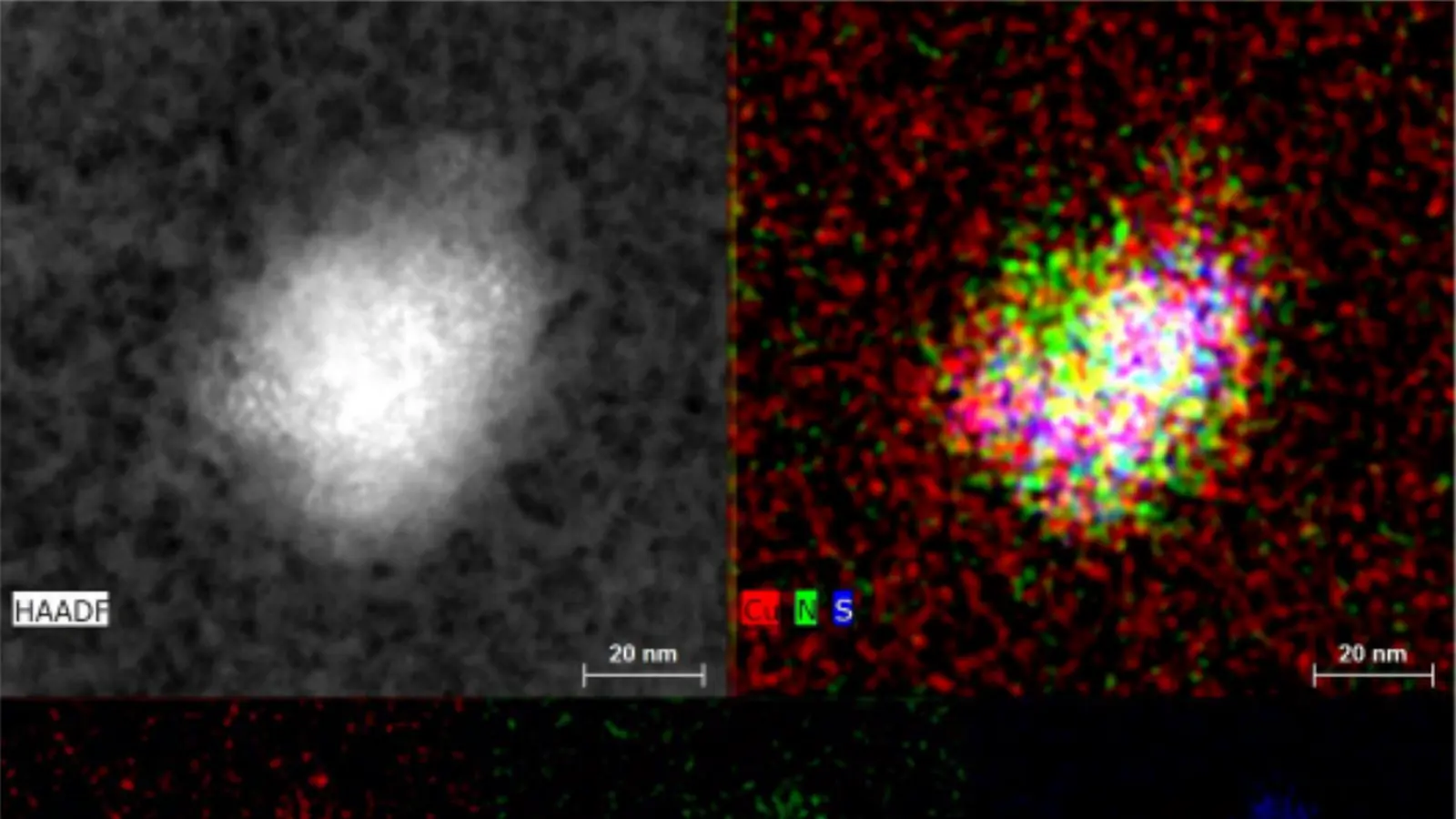
The NaDiNa drug candidate is an anticancer agent based on a nanotechnology formulation of the copper salt of diethyldithiocarbamate (CuET), utilizing albumin as a carrier. The product was developed by UPOL scientists for intravenous administration and targeted treatment of solid tumors and hematological malignancies. Its mechanism of action lies in the specific binding of CuET to the protein NPL4, which induces proteotoxic stress and leads to cancer cell death. The results of this long-term research were published in the prestigious scientific journal Nature (December 14, 2017; Vol. 552, Issue 7684, pp. 194–199).
Project Progress and Results
The project had two main directions. Our company focused on the Process development of the dosage form under GMP conditions, while UPOL conducted the preclinical testing of the drug.
The development led by the Institute of Molecular and Translational Medicine (IMTM) at the UPOL Faculty of Medicine included optimization of the nanoparticle formulation and stability tests in infusion solutions. As part of the preclinical studies, pharmacokinetics, toxicity, and efficacy were evaluated in tumor in vivo models. In addition, a GLP-certified method for the determination of CuET in biological material was developed.
In vitro experimental data confirm the selective cytotoxic effect against tumor cells, including cells resistant to conventional chemotherapy. The drug thus represents a promising candidate for further preclinical and clinical development in oncology. Another significant achievement is the granting of several national patents covering the preparation of albumin–CuET nanoparticles, including protection in the United States.
Among the most important outputs on the part of oncomed are not only the development of the formulation and the optimization of the manufacturing process of the drug, but above all the production of a clinical batch of CuET, including complete documentation enabling its use for Phase I clinical trials. An integral part of the project was also the development and implementation of analytical methods necessary for testing the manufactured batch, as well as the preparation of manufacturing and technical documentation ensuring reproducible production and its use in further clinical development and contract manufacturing.
Official Approval
The project was successfully defended on July 8, 2025, as part of the final opposition procedure, and subsequently approved for closure by the TAČR Presidium on August 7, 2025.
“The official evaluation by the Technology Agency of the Czech Republic confirmed that the project achieved results corresponding to its defined objectives and created a solid foundation for further clinical development of the drug and its potential use in practice,” said Radek Fialka, Commercial Director at medac CDMO.
Conclusion
The implementation of the project has demonstrated our ability to effectively connect academic research with industrial manufacturing and deliver results with significant potential for the further advancement of modern medicine.
Complete list of publications and sources cited in Annex 15 – Loffelmann (European Journal of Medicinal Chemistry, 2023). All are listed, including DOI/links:
References – Loffelmann et al., EJMECH 2023
1. Boguski MS, Mandl KD, Sukhatme VP. Repurposing with a difference. Science (2009). https://doi.org/10.1126/science.1169920
2. Liu P, Brown S, Goktug T, et al. Cytotoxic effect of disulfiram/copper on human glioblastoma… Br J Cancer (2012). https://doi.org/10.1038/bjc.2012.442
3. Liu X, Wang L, Cui W, et al. Targeting ALDH1A1 by disulfiram/copper… Oncotarget (2016). https://doi.org/10.18632/oncotarget.11305
4. Skrott Z, Majera D, et al. Disulfiram’s anticancer activity reflects targeting NPL4… Oncogene (2019). https://doi.org/10.1038/s41388-019-0915-2
5. Johansson B, Stankiewicz Z. Bis-(diethyldithiocarbamato) copper complex… Biochem Pharmacol (1985). https://doi.org/10.1016/0006-2952(85)90026-7
6. Lipsky JJ, Shen ML, Naylor S. In vivo inhibition of aldehyde dehydrogenase by disulfiram. Chem Biol Interact (2001). https://doi.org/10.1016/S0009-2797(00)00225-8
7. Lam JP, Mays DC, Lipsky JJ. Inhibition of human mitochondrial ALDH… Biochemistry (1997). https://doi.org/10.1021/bi970948e
8. Hart BW, Faiman MD. Bioactivation of disulfiram metabolites. Biochem Pharmacol (1993). https://doi.org/10.1016/0006-2952(93)90619-8
9. Skrott Z, Cvek B. Mechanism of action in cancer cells. Mini-Rev Med Chem (2012). https://doi.org/10.2174/138955712802762068
10. Skrott Z, Mistrik M, Andersen KK, et al. Disulfiram targets cancer via p97 segregase adaptor NPL4. Nature (2017). https://doi.org/10.1038/nature25016
11. Meyer H, Bug M, Bremer S. Functions of VCP/p97 AAA-ATPase… Nat Cell Biol (2012). https://doi.org/10.1038/ncb2407
12. Deshaies RJ. Proteotoxic crisis and cancer therapy. BMC Biol (2014). https://doi.org/10.1186/s12915-014-0094-0
13. Chroma K, Skrott Z, Gursky J, et al. Drug repurposing strategy for multiple myeloma. Cell Death Dis (2022). https://doi.org/10.1038/s41419-022-04651-w
14. Pan M, Zheng Q, Yu Y, et al. Seesaw conformations of Npl4 in p97 complex. Nat Commun (2021). https://doi.org/10.1038/s41467-020-20359-x
15. Majera D, Skrott Z, et al. Targeting NPL4 by disulfiram evokes replication stress. Cells (2020). https://doi.org/10.3390/cells9020469
16. Kanellis DC, Zisi A, Skrott Z, et al. Cancer vulnerability due to translational arrest and p53 aggregation. Cell Death Differ (2023). https://doi.org/10.1038/s41418-023-01167-4
17. Woerner AC, Frottin F, Hornburg D, et al. Protein aggregates interfere with nucleocytoplasmic transport. Science (2016). https://doi.org/10.1126/science.aad2033
18. Kaul L, Süss R, Zannettino A, Richter K. The revival of dithiocarbamates. iScience (2021). https://doi.org/10.1016/J.ISCI.2021.102092
19. Hogarth G. Metal-dithiocarbamate complexes: chemistry and biological activity. Mini Rev Med Chem (2012). https://doi.org/10.2174/138955712802762095
20. Chen D, Peng F, Cui QC, et al. Pyrrolidine dithiocarbamate-copper complex inhibits proteasome in prostate cancer. Front Biosci (2005).
21. Lövborg H, Öberg F, Rickardson L, et al. Disulfiram inhibits proteasome activity… Int J Cancer (2006). https://doi.org/10.1002/ijc.21534
22. Buchtova T, Skrott Z, Chroma K, et al. Cannabidiol impedes anticancer effects of disulfiram. Mol Oncol (2022). https://doi.org/10.1002/1878-0261.13114
23. Fujie T, Murakami M, Yoshida E, et al. Copper diethyldithiocarbamate as an activator of Nrf2. J Biol Inorg Chem (2016). https://doi.org/10.1007/s00775-016-1337-z
24. Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell (2010). https://doi.org/10.1016/j.molcel.2010.10.006
25. Guettouche T, Boellmann F, Lane WS, Voellmy R. Phosphorylation of HSF1 under stress. BMC Biochem (2005). https://doi.org/10.1186/1471-2091-6-4
26. Majera D, Skrott Z, Bouchal J, et al. Targeting stress-response pathways by PARP inhibitors, vorinostat and disulfiram. Prostate (2019). https://doi.org/10.1002/pros.23741
27. Biamonti G, Vourc’h C. Nuclear stress bodies. Cold Spring Harb Perspect Biol (2010). https://doi.org/10.1101/cshperspect.a000695
28. Hetz C. The unfolded protein response. Nat Rev Mol Cell Biol (2012). https://doi.org/10.1038/nrm3270
29. Cvek B, Milacic V, Taraba J, Dou QP. Ni(II), Cu(II), Zn(II) diethyldithiocarbamate complexes in cancer cells. J Med Chem (2008). https://doi.org/10.1021/jm8007807
30. Chen W, Yang W, Chen P, et al. Disulfiram copper nanoparticles for prostate cancer. ACS Appl Mater Interfaces (2018). https://doi.org/10.1021/acsami.8b14940
31. Paun RA, Dumut DC, Centorame A, et al. Nanoliposomal copper diethyldithiocarbamate in cancer therapy. Pharmaceutics (2022). https://doi.org/10.3390/pharmaceutics14030640
32. Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol (2017). https://doi.org/10.1038/nrclinonc.2016.206
33. Klöpping HL, van der Kerk GJM. Investigations on organic fungicides IV. Recueil des Travaux Chimiques des Pays-Bas (1951). https://doi.org/10.1002/RECL.19510701008
34. Gohei H. Heavy-metal immobilization agents. Patent JP10076240 A (1998).
35. Konečný V. Synthesis and biological properties of dithiocarbamic acid derivatives. Chem Zvesti (1984).
36. Afzaal M, Ellwood K, Pickett NL, et al. Growth of lead chalcogenide thin films… J Mater Chem (2004). https://doi.org/10.1039/B313063K
37. Chandra K, Garg AK, Jain MC, et al. Bis-dithiocarbamato complexes of bis-biphenyl tin(IV). J Indian Chem Soc (1980). https://doi.org/10.5281/ZENODO.6361397
38. Tanaka Y, Odo J, Kariya K. Chelatometric titration of mercury(II). Bunseki Kagaku (1977).
39. Thomson JF, Savit J, Goldwasser E. Tests of dithiols for decontamination of lewisite. J Pharmacol Exp Therapeut (1947).
40. Hayano K, Yoneyama H, Hayashi Y. Syntheses of piperazines I. Yakugaku Kenkyu (1958).
41. Watanabe Y, Hirai K. Fungicidal dithiocarbamates. Patent DE2224597 A (1972).
42. Kreider EM. 2-(2-Methyl-5-nitro-1-imidazolyl)ethyl-N-arylalkyldithiocarbamates. Patent US3910945 A (1975).
Annex 17 – Patent (US 11,766,404 B2) has its own bibliography, the official application is available here: https://patents.google.com/patent/US11766404B2
From patent US 11,766,404 B2 (Annex 17) – complete list:
Patent citations
• US20170143729A1
• US20190337106A1
• WO2017207317A1
• WO2018202283A1
• EP3402581A1
• CN110293998A
Non-patent literature
1. Skrott Z. et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 552 (2017): 194–199. https://doi.org/10.1038/nature25016
2. Cvek B. Diethyldithiocarbamate complex with copper: the mechanism of action in cancer cells. Mini-Rev Med Chem 12 (2012): 1184–1192. https://doi.org/10.2174/138955712802762068
3. Liu P. et al. Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer stem-like cells. Br J Cancer 107 (2012): 1488–1497. https://doi.org/10.1038/bjc.2012.442
4. Kanellis D.C. et al. Actionable cancer vulnerability due to translational arrest, p53 aggregation and ribosome biogenesis stress evoked by the disulfiram metabolite CuET. Cell Death Differ (2023). https://doi.org/10.1038/s41418-023-01167-4
5. Majera D. et al. Targeting the NPL4 adaptor of p97/VCP segregase by disulfiram as an emerging cancer vulnerability evokes replication stress and DNA damage while silencing the ATR pathway. Cells 9 (2020): 469. https://doi.org/10.3390/cells9020469
6. Kaul L. et al. The revival of dithiocarbamates: from pesticides to innovative medical treatments. iScience 24 (2021). https://doi.org/10.1016/j.isci.2021.102092